The Ultimate Guide On Metal Surface Treatments
Upload Time:
Feb 24, 2024
At times, you would like to alter sheet metal surfaces. In such situations, you may wish to process metal surface in different ways. This guide will help you choose a suitable process for superior surface quality. Keep reading to learn more.
Several reasons can necessitate changing the surface characteristics of metals through treatment. Some of these reasons are as follows:
- Enhance resistance to corrosion.
- Increase hardness for wear resistance.
- Repair damage on the metal surface.
- Provide certain desired features to ordinary metals.
- For beauty and aesthetic purposes.
Some of the treatments you employ on metal surfaces are:
Metal Peening
In peening, you employ a shot blast or laser focus onto the metal surface to increase its resistance to fatigue. The blast’s abrasive media hits the metal surface at high velocity causing minimal plastic deformation useful in maintaining residual tension.
Advantages
- Reduces residual stress resulting from welding, casting and forging processes.
- Facilitates descaling and surface extinction.
- Improves the metal’s mechanical strength.
- Enhances material’s resistance to wear, fatigue and corrosion.
Disadvantages
- Can require pre-processing which can increase the process time.
- If done without caution, it can cause undesired roughness.
Application
- Used in vehicle parts such as coil springs, axles and leaf springs for fatigue resistance.
- Making torsion bars in industrial equipment.
- Drilling machines employed in fossil fuel extraction operations.
- Turbine blades and high performance pressure compressors.
Best Suited Metals
- Aluminium alloy
- Carbon steel and low alloy steel
- Ductile iron
- Nickel alloy
- Titanium alloy
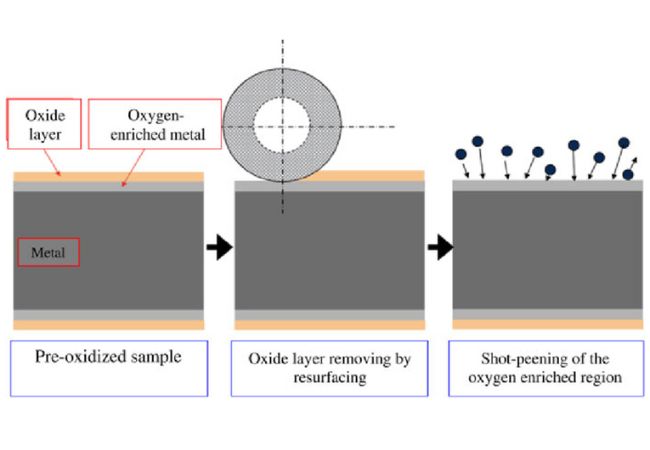
Surface Preparation Before Metal Peening in Metal Surface Treatment
Metal Polishing
Metal polishing exhibits the least invasive of the metal surface treatments and can be mechanical or chemical. It employs an abrasive material to remove blemishes from the metal surface leaving it smooth and shiny. It typically begins with a preliminary stage involving thorough cleaning followed by descaling and polishing.
Advantages
- Leaves metal surfaces with a smooth and shiny surface.
- Is a low cost process that doesn’t require skilled operators.
- Prevents oxidation and surface contamination.
- Reduces wear of the surface.
Disadvantages
- Requires use of abrasive particles.
- The resulting surface is not permanent and can lose its luster.
- May need a post process after polishing such as buffing to enhance appearance.
Application
- Used in automobile parts such as body panels.
- Pharmaceutical and dairy containers employ polishing to minimize corrosion and occurrence of mold.
- Kitchenware such as aluminum pans and pots.
- Manufacturing of light reflectors.
Best Suited Metals
- Aluminum
- Brass and bronze
- Copper and beryllium copper
- Stainless, low and high carbon Steels
- Titanium
Metal Burnishing
Burnishing chemically blackens metal parts enhancing appearance and protecting the surface from corrosion. The metal turns into a shiny hue of blue-black appearance after blackening without altering thickness.
Burnishing employs lead acetate or lead acetate enhanced water for ferrous materials except steel or certain oils for iron and steel.
Advantages
- Has low control needs taking little time to execute.
- Involves no material removal.
- As a chemical process it has low noise.
- The resulting surface is long lasting.
- Does not alter the dimensions of the metal part.
Disadvantages
- The slow rate of production does not favor high volume production.
- Burnishing is inapplicable for small parts.
- Is difficult to undertake with complex designs.
Application
- Treatment of turbine blades and airfoils.
- Furnishing equipment in the food and medical industries.
- Finishing cutting tools like grinders.
Best Suited Metals
- Iron
- Brass
- Aluminum
- Carbon steel
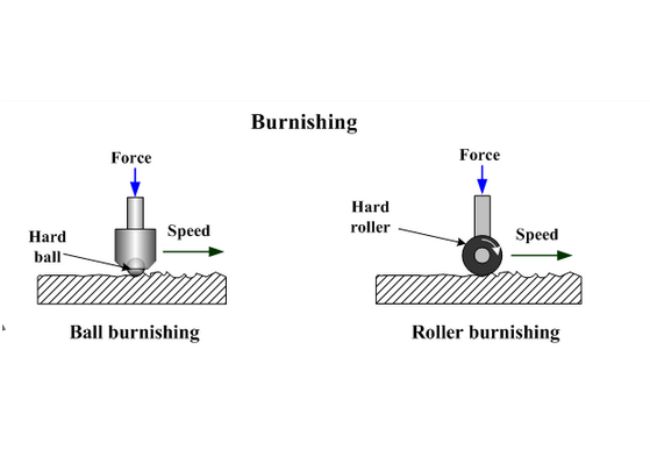
How Metal Burnishing is Done
Metal Phosphatization
Phosphatization is a treatment for metal surfaces where you chemically modify the surface using phosphate crystals. You can employ zinc, manganese or iron phosphate crystals to improve the wear and corrosion resistant quality of the metal.
Advantages
- Offers great corrosion and wear resistance.
- Can function as a surface preparation for additional process such as coating.
- The resulting layer’s abrasiveness can function as lubricating layer when performing cold work.
Disadvantages
- Phosphatization is poor on non-ferrous metals such as copper and brass.
- The resulting coating is usually porous requiring filling.
- It is a technical process that requires skilled application.
Application
- Finds use as in pre-treating steel sheets prior to painting in automotive applications.
- Its use on firearms is a better alternative to results in a better finish compared to bluing.
Best Suited Metals
- Zinc
- Cadmium
- Silver
- Tin
- Steel
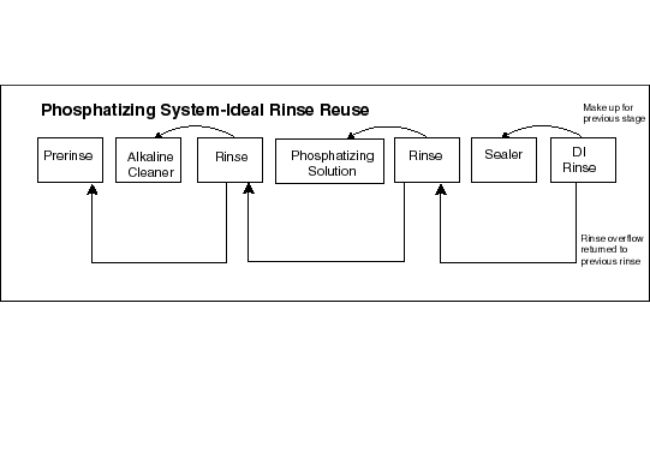
Metal Phosphatization Operation
Electroplating Metal
Electroplating applies a layer of metal (anode) onto another metal surface (cathode) by an electrochemical process. The applied metallic layer typically exists in an electrolyte solution with the application of a direct source of current.
Advantages
- Creates a protective barrier layer for substrate metal against wear, heat, impact and corrosion.
- Elicits friction resistance especially with nickel plating.
- Augments the properties of substrate metal such as conductivity.
- Enhances adhesion between the substrate and coating creating a firm surface.
- Alters the thickness of the surface offering more protection.
Disadvantages
- The electroplating process takes time due to the slow deposition process.
- The process results in waste material which can cause pollution if carelessly deposed.
Application
- Finds use in jewelry design to attain an ornamental finish.
- Aesthetically customizing vehicle parts usually by adding chromium.
- Used in electronic devices to enhance conductivity.
- Fabricating switchgears n the telecommunications sector by applying gold or palladium.
Best Suited Metals
- Zinc
- Copper
- Tin
- Gold
- Silver
- Palladium
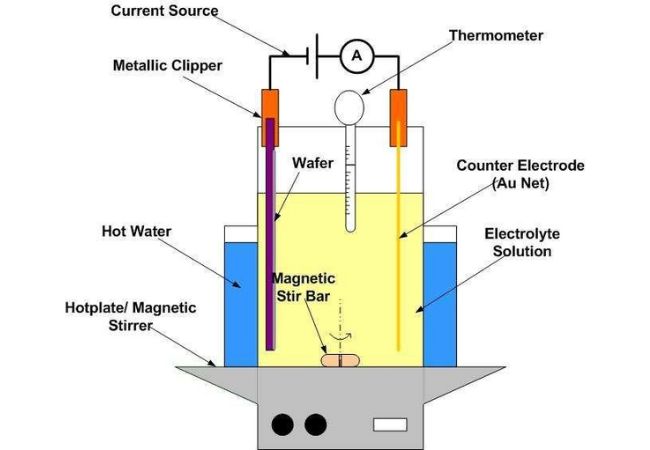
Electroplating in Metal Surface Treatment Overview
Anodizing Metal
Anodizing commonly finds use on aluminum and its alloys involving electrolytic submersion and external current application to produce resistant coating. The workpiece acts as anode resulting in a film tightly bonded to the substrate.
Advantages
- Anodized metal surfaces are highly durable offering you an extended service life.
- The resultant coating requires less maintenance needs given its impressive resistant qualities.
- Anodizing results in an aesthetically pleasant finish with possibility of different colours.
- The overall cost of applying and maintaining an anodized surface treatment is relatively low.
- The substances employed in anodizing and process itself are not hazardous.
Disadvantages
- It does not work on steel and limited only to specific metals commonly certain aluminum grades.
- When undertaking large volume productions, it is difficult to maintain consistency.
- It is uneconomical for small volume productions.
Application
- Furnishing exteriors and roofing systems for buildings.
- Home appliances like washers, refrigerators and microwaves employ anodized aluminum.
- Furniture and equipment such as tables, storage cabinets and lockers.
- Food industry equipment like grills, burner sets, pans and display cabinets.
- Boats and water vessels such as canoes and kayaks.
- Body panels for aircrafts and vehicle parts such as name plates and wheel caps.
Best Suited Metals
- Aluminum and its alloys
- Titanium
- Niobium
- Zirconium
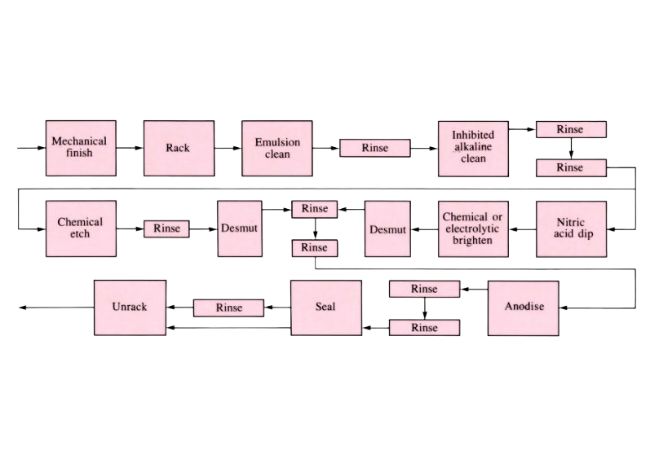
Operations Employed in Anodizing
Conversion Coatings
Conversion coatings involve the chemical or electro-chemical subjection of metal parts transforming the surface into a thin, adherent and insoluble layer. There are different conversion coatings you can employ such as oxide coating and chromate coating.
Advantages
- Produces an impressive dark finish that is corrosion resistance.
- Ensures minimal dimensional changes.
- Is a long lasting surface treatment.
- Improves the lubrication quality of the metal surface.
- Its dark hue reduces reflection.
Disadvantages
- The resulting hue can change.
- Some application encounter issues to do with coverage.
- The surface is susceptible to wear by aggressive staining.
Application
- Automotive parts such as hood fasteners employ conversion coatings.
- Conversion coating finds use on firearm barrels.
- Home and garden tools and equipment like shovels and grills.
Best Suited Metals
- Cadmium
- Tin
- Aluminum
- Zinc
- Copper
- Stainless steel
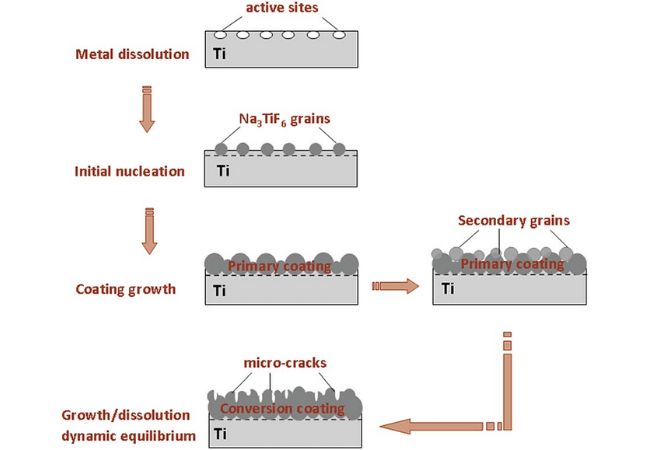
Conversion Coating
Thermal Spraying
Thermal spraying involves heating and melting materials and dispersing them on workpiece surface using compressed air. This results in a firm coating with highlighted qualities relating to corrosion, wear and heat tolerance.
Advantages
- You can employ different materials for thermal spraying including metals and non-metals.
- The resultant coating offers a long service life.
- Thermal sprayed coatings are cheap to apply.
- There is no grand heat input when using thermal spraying.
- You can achieve different thickness depending on application.
Disadvantages
- It completely coats the metal substrate making identification difficult.
- Thermal spraying is an expensive set-up involving costly equipment.
Application
- Treating aerospace components such as turbine blades and combustion chambers.
- Moving parts of heavy industry machinery.
- Drilling bits ant tools used in exploration in the petroleum industry.
- Hydraulic piston rods and electric motors.
Best Suited Metals
- Aluminum
- Molybdenum
- Iron
- Nickel
- Chromium and cobalt
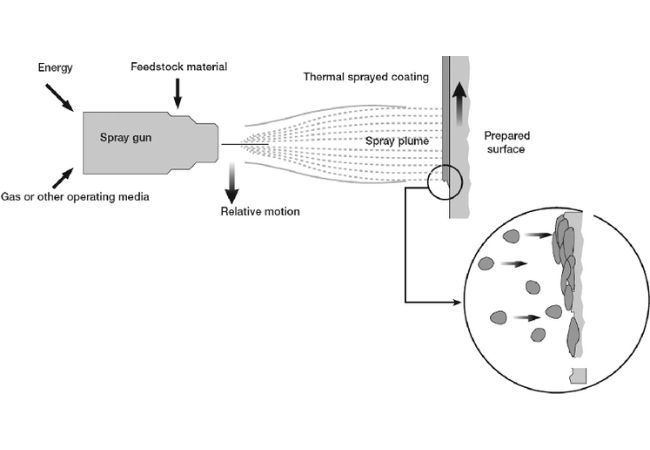
Thermal Spraying Process
Annealing
Annealing is a surface treatment process that changes mostly physical characteristics of the metal by heat application. Some of the properties changed include hardness and ductility which increase machinability.
In annealing, you raise a material’s temperature above its recrystallization point, hold it there for some time, and then cool. You define this processes in stages as the recovery stage, recrystallization and grain growth.
Advantages
- Annealing increases a material hardness and decreases brittleness.
- Eliminates the occurrence of internal stresses.
- Enhances ductility improving workability.
- Boosts a material’s magnetic qualities.
- You have the option of different techniques to imbue certain qualities.
Disadvantages
- Weakens the material’s conductivity resistance.
- Undertaking an annealing process is relatively costly.
- The grain size achieved in annealing is not uniform.
- Annealing is time invasive.
Application
- Gear manufacturing in mechanical industries.
- Repairing disorders in semiconductors such as ion implantation.
- Metal fabrication in mills.
- Tools and equipment such as knives and blades.
- Springs for use in vehicles and machines.
Best Suited Metals
- Steel
- Cast iron
- Aluminum
- Copper
- Brass
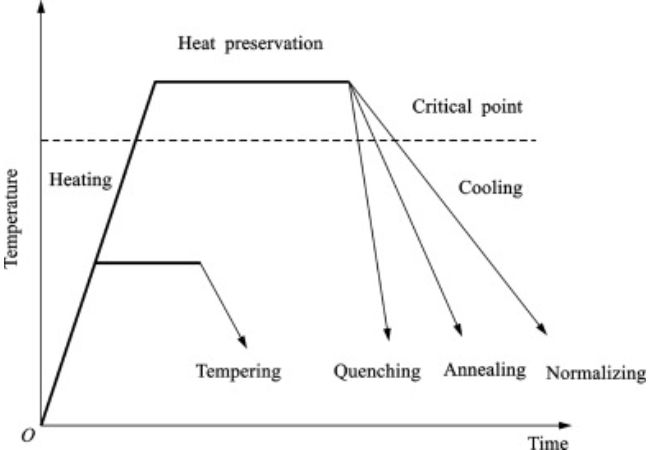
Annealing Graphic Overview
Relevant News









